Imagine a day without batteries – no mobile phones, no remote-controlled toys, and no flashlights. Batteries are essential in our daily lives, yet we often overlook their importance and the science behind them. Let’s dive into the world of batteries to understand what they are, how they work, and their different types.
What is a Battery?
A battery is like a tiny power station you can hold in your hand. It doesn’t create electricity but stores it in a form that we can use whenever we need. Just like a water tank stores water, a battery stores electrical energy. This stored energy is in the form of chemical energy, which can be converted into electrical energy to power various devices, from remote controls to smartphones.
Components of a Battery
Understanding a battery’s inner workings can be fascinating. Here are its key components-
Cells-
- Each battery is made up of one or more cells.
- Anode (Negative Side)- This is where the electricity comes from. When the battery is used, chemical reactions start at the anode, releasing electrons (tiny particles that carry electricity).
- Cathode (Positive Side)- The positive side where electrons flow to. The cathode attracts electrons, completing the circuit and allowing the battery to power a device.
- Electrolyte– A chemical substance (can be liquid, paste, or solid) that sits between the anode and cathode. It allows ions (electrically charged atoms or molecules) to move between the anode and cathode, facilitating the chemical reactions that produce electricity.
Terminals-
- Terminals are the points on a battery that connect to the device it powers.
- The positive terminal (+) is usually marked and connects to the cathode. The negative terminal (-), connecting to the anode, completes the circuit when the battery is in use.
- When you put batteries into a device, ensuring that these terminals match the device’s corresponding positive and negative signs is essential.
How Does a Battery Work?
Understanding how a battery works can be as exciting as solving a mystery. Let’s break it down-
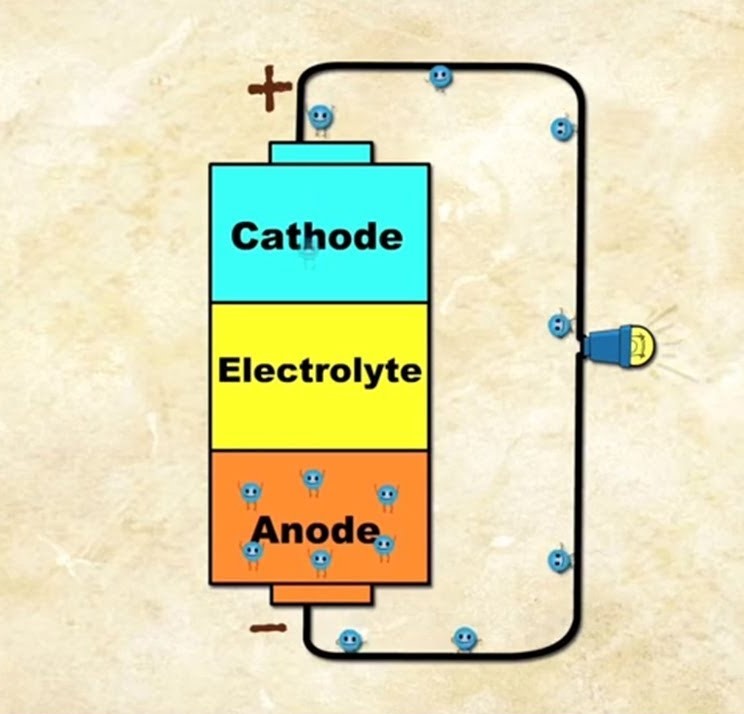
Chemical Reaction Inside a Battery-
- Inside each battery cell, there’s a mini science lab where a chemical reaction happens.
- This reaction involves the battery’s components- the anode, cathode, and electrolyte.
Role of the Anode and Cathode-
- The anode (negative side) and cathode (positive side) are like two different worlds in a battery.
- During the reaction, the anode undergoes a transformation, releasing electrons. Electrons are super tiny particles, much smaller than even the smallest ant you’ve ever seen.
Journey of Electrons-
- These electrons start to move from the anode towards the cathode. Why? Because electrons are negatively charged and the cathode is positively charged. Opposites attract!
- The electrolyte acts as a bridge in this journey. It’s a substance that helps move the electrons from the anode to the cathode.
Creation of Electric Current-
- This movement of electrons from the anode to the cathode is what we call an electric current. It’s like a tiny river of energy flowing within the battery.
- When you connect a battery to something like a toy or a flashlight, this current flows into the device.
Powering the Device-
- The flow of electrons through the device is what powers it up. It’s like the battery is telling the toy, “Here, take some of my energy!”
- Once the electrons reach the cathode, the reaction slows down, and eventually, the battery runs out of energy.
Different Types of Batteries
Single-Use Batteries (Primary Batteries)-
- Description- These are batteries that you use once and then dispose of. They cannot be recharged once they run out of energy.
- Examples- The AA or AAA batteries in toys and TV remotes are single-use. They are convenient for devices that need battery replacement less often.
- Chemistry Involved- Most common single-use batteries are alkaline batteries. They contain zinc and manganese dioxide.
Rechargeable Batteries (Secondary Batteries)-
- Description- Unlike single-use batteries, these can be recharged and used multiple times.
- Examples- Batteries in your smartphones, laptops, and rechargeable toothbrushes are typically rechargeable batteries.
- Chemistry and Technology- They often use lithium-ion or nickel-cadmium technology, enabling them to store and release energy multiple times.
Series and Parallel Connections
Series Connection-
- How it Works- When you connect the positive terminal of one cell to the negative terminal of another, it’s a series connection.
- Effect on Voltage- This setup increases the total voltage. For instance, connecting two 1.5-volt cells in series gives you a total of 3 volts.
- Practical Use- Useful in devices that require a higher voltage than what a single cell can provide.
Parallel Connection-
- How it Works– Here, you connect all the positive terminals together and all the negative terminals together.
- Effect on Capacity- This doesn’t increase the voltage but increases the total capacity, meaning the batteries last longer.
- Practical Use- Ideal for devices like TV remote controls where longer battery life is more important than higher voltage.
Common Uses of Batteries
- In Portable Devices- Batteries power many portable devices like flashlights, handheld games, and remote controls, making them convenient to use anywhere.
- In Automobiles- Car batteries, which are rechargeable, help start engines and power the electrical system of the vehicle.
- In Renewable Energy Systems- Batteries are essential in systems like solar panels, where they store solar energy for use when the sun isn’t shining.
Safety and Recycling
It’s important to use batteries safely, as they contain chemicals that can be harmful. Always dispose of batteries properly, and remember that recycling them helps protect the environment.
Batteries are fascinating and powerful tools that keep our world running. From powering simple toys to complex machinery, they are a cornerstone of modern technology. By understanding how batteries work, you can appreciate the science that lights up your life every day.
Also Check=Electromagnets- A Guide for Upper Primary Students
ALso Check – Electric Bell Working Mechanism Simplified for Students