Air Around Us
Air is very important to us. We cannot live without air even for a few minutes. It is all around us, we take it in when we breathe. Air consists of a mixture of gasses like oxygen, nitrogen, helium etc. it also contains bacteria, viruses. The thick layer of air that surrounds the earth is called the atmosphere. Earth’s gravity holds the atmosphere around the Earth. It protects us from the harmful Ultraviolet rays of the Sun.
The Atmosphere
The several hundred kilometers thick layer formed by the air around the Earth is called the atmosphere. The density of air decreases with height. Air density is greatest at the surface, and decreases as we move up in the atmosphere because the weight of air above becomes less. The atmosphere plays a major role in protecting us from meteoroid hits by burning them up in the mesosphere before they can hit the surface of the earth. It also protects us from harmful UV rays.
5 Important Layers of the Atmosphere
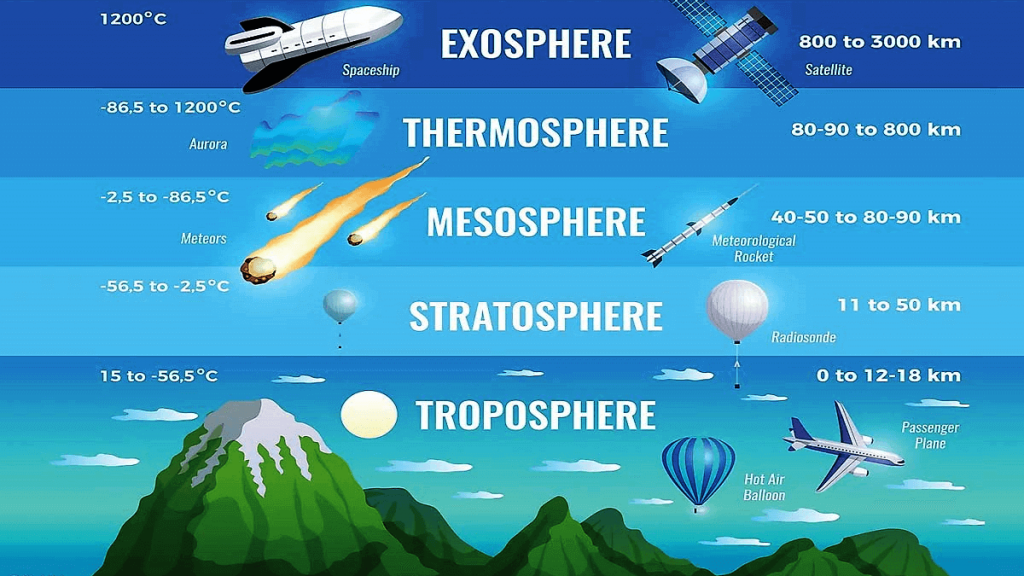
5 Important layers of atmosphere are :
- The Troposphere
- The Stratosphere
- The Mesosphere
- The Thermosphere
- The Exosphere
The Troposphere :
- The lowest layer nearest to the Earth’s surface is called the troposphere.
- It extends upto about 15km above the surface.
- Humans live in the troposphere.
- Most weather changes occur in this layer.
- Clouds are formed in this layer because 99% of the water vapor in the atmosphere is found in the troposphere.
- The Air gets colder as you climb higher in the troposphere.
The Stratosphere:
- The second layer of the atmosphere is called the Stratosphere.
- It extends from the top of the troposphere to about 50km above the surface.
- The ozone layer is found in this layer.
- Unlike the troposphere, the stratosphere actually gets warmer the higher you go.
- Commercial jet planes usually fly in this layer.
The Mesosphere
- The layer that Lies above the stratosphere is called mesosphere.
- It extends to about 85-90 km about Earth’s surface.
- Most meteors burn up in this layer.
- Temperatures once again grow colder as you rise up through the mesosphere.
The Thermosphere
- The layer that Lies above the mesosphere is called the thermosphere.
- It extends to about 500-600 km above Earth’s surface.
- High-energy X-rays and UV radiation from the Sun are absorbed in the thermosphere.
- The layer of the air is very thin in this layer.
- This layer absorbs High-energy X-rays and UV radiation from the Sun.
- Many satellites orbit Earth within this layer
The Exosphere
- The layer that Lies above the thermosphere and fades into space is called the exosphere.
- It extends somewhere between 100,000 km and 190,000 km above the surface of Earth.
The Importance of the Atmosphere
- It contains oxygen , which all living organisms need to survive.
- It has carbon dioxide which is needed by the plants for the process of photosynthesis.
- Air is needed for burning as it contains oxygen which helps in combustion.
- It has a layer of gas called the ozone layer which is present in the strastopsphre which protects us from harmful UV rays of the Sun thus protecting us from many skin diseases and eye problems.
- Gasses present in the atmosphere reflect or absorb the strongest rays of sunlight.
- It also moderates the Earth’s temperature.
- Atmosphere also protects us from meteoroids.
What does Air Contain
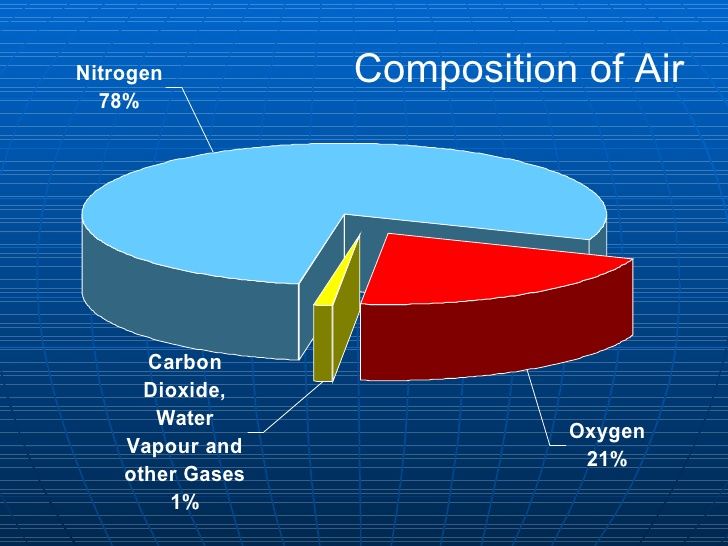
The air around us is a mixture of several gasses. It contains 78% nitrogen, 21% oxygen and 1% mainly argon and 0.03% carbon dioxide. In addition to these gases, air also contains some amount of water vapor, dust and smoke. Germs are also present in the air.
Oxygen:
- Oxygen is the most important gas needed for the survival of all living organisms.
- Oxygen forms ⅕ th of the Earth’s atmosphere.
- It is one of the main elements that is present in the air.
Nitrogen:
- Nitrogen is the most abundant element on our planet.
- It has two molecules of nitrogen.
- Plants use nitrogen with the help of bacteria present in the soil.
- Animals get nitrogen from plants, fish and meat.
Carbon Dioxide :
- It is a colorless, odorless gas present in the atmosphere which is important for the plants for the process of photosynthesis.
- It is an important greenhouse gas which helps to trap heat in the atmosphere.
Other Gasses:
- Some percentage of other gasses like hydrogen, helium, neon, argon and krypton are also present in the air.
- Argon is a colorless and odorless gas present in air. It is used in making light bulbs and fluorescent tubes.
Water Vapour :
- Water vapour is the source of moisture for clouds, rain and frost.
- It is formed because of evaporation of water from the surface of water bodies.
- The amount of water vapour present in the air is called humidity.
- Water vapour absorbs heat in the lower atmosphere and radiates the heat in all directions.
Properties of Air Air Occupies Space
Air is everywhere around us. We are not able to see the air but it is present everywhere from an empty cup to an empty room.
Experiment to prove that air occupies air

Take a balloon and blow air into it.
You will notice that the size of the balloon increases.
This shows that air takes up space.
Air Has weight
Even though we cannot feel it, air has some weight.
Anything with mass has weight and air has mass because we can feel it when the wind blows.
Experiment to prove that Air has weight
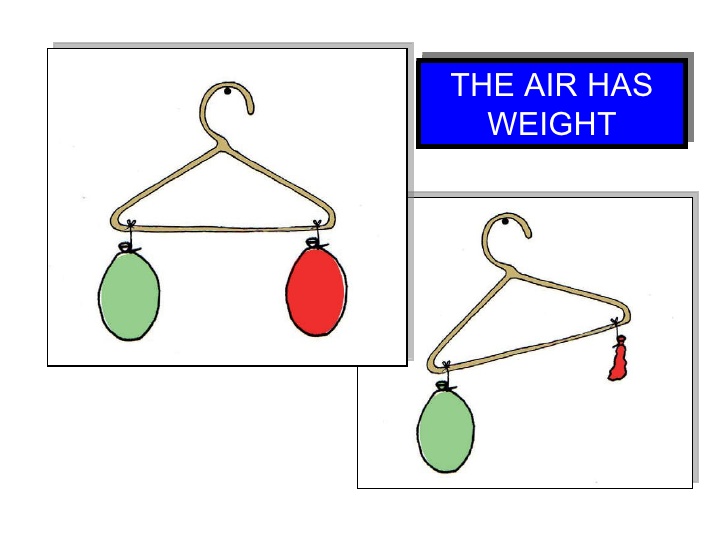
- Take two balloons (green and red ).
- Fill the two balloons with equal amounts of air.
- Tie each balloon at the ends of the stick or hanger respectively.
- Now deflate the red balloon.
- The green balloon will drop down as the stick will tilt down towards the air filled balloon because of the weight of the air inside it.
Air Exerts Pressure
Air pressure is the force with which air pushes against the Earth’s surface. Air has density and mass. Due to its mass it puts pressure on the things around it.
Experiment to Prove that air exerts Pressure
- Take a glass of water and fill it completely with water.
- Cover the mouth of the glass with cardboard making sure there are no bubbles in the water below the cardboard.
- Now press the cardboard with your hand and invert the glass and remove your hand from the cardboard.
- The cardboard will not fall as the air exerts pressure on the card to keep it in place.
Air Exerts Pressure in the upward direction
Experiment to Prove Air Exerts Pressure in the Upward Direction
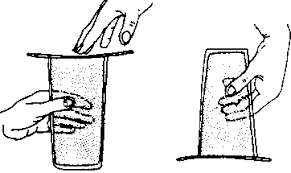
- Take a glass of water and fill it with water to the brim
- Cover the tumbler with a stiff paper.
- Press the paper with your hand and turn it upside down.
- We will see that the paper will not fall and water stays in the glass.
- The air pushes the paper up,i.e, exerts pressure in the upward direction preventing it from falling.
Air Exerts pressure in Downward direction
Experiment to Prove Air Exerts pressure in Downward direction
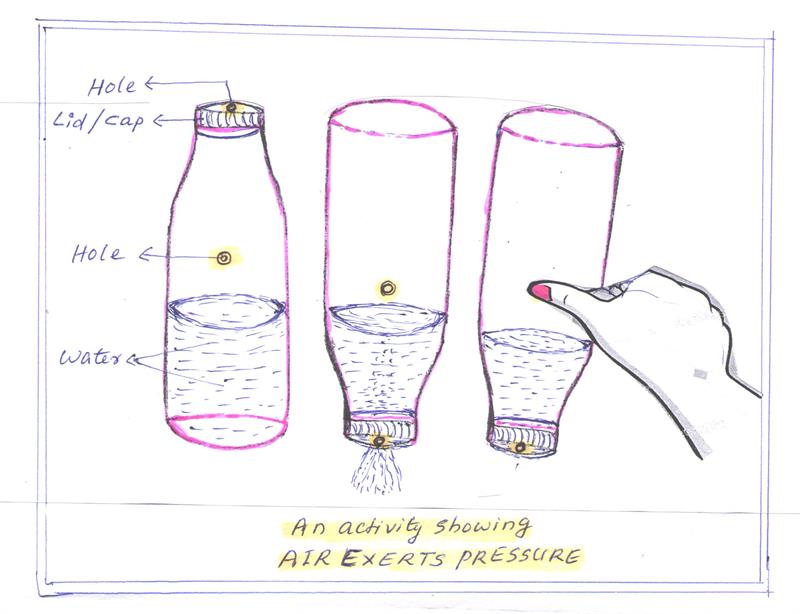
- Take a plastic bottle filled with water and close the cap tightly .
- With the help of a pin make a hole on the side of the bottle.
- We will see that the water will not come out of the hole.
- Now open the cap of the bottle .
- We will see that the water will come out of the hole because air exerts pressure in a downward direction.
Air is needed for burning
Experiment to prove that air is needed needed for Burning
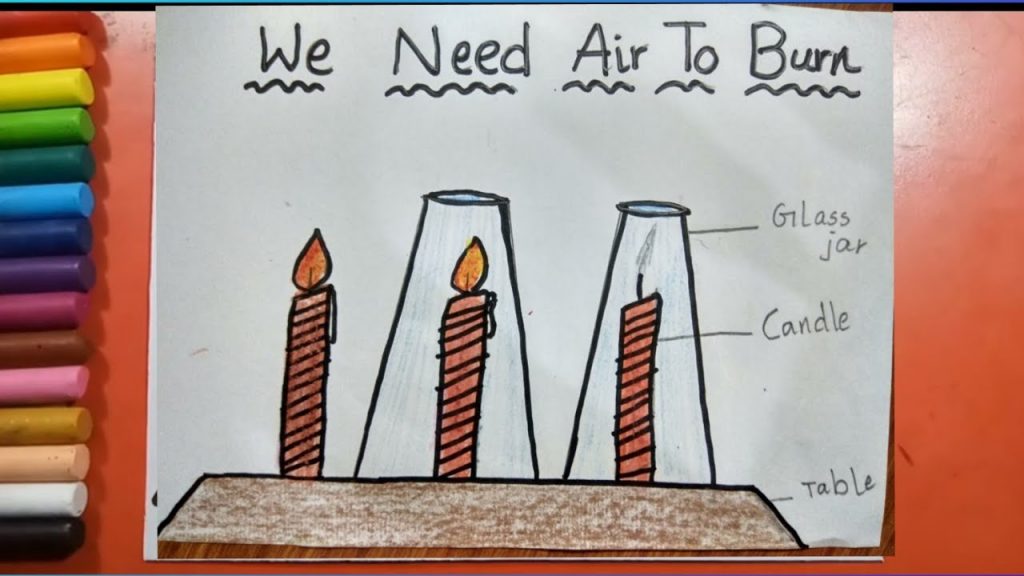
- Take a candle and light it.
- The candle will continue to burn due to the air present around it .
- Cover the candle with a lid now .
- After sometime we will see that the candle will stop burning.
- This proves that air is needed for burning as gasses like oxygen present in air helps in combustion.
Atmospheric pollution
- Air contains particles of dust, smoke and harmful gasses. They are called atmospheric pollutants as they make the air dirty and polluted.
- Atmospheric pollutants are substances that accumulate in the air to a degree that is harmful to living organisms or to materials exposed to the air.
- Atmospheric pollution is a big threat which leads to a number of diseases.
- Burning of fossil fuels, gasses released by automobiles, factories & industries release smoke and harmful gasses in the air which degrade the quality of the air .
- Atmospheric pollution can lead to the occurence of a number of respiratory disorders and heart diseases among humans.
- It also leads to global warming.
Water
About 71% of the Earth’s surface is covered with water. It is found everywhere from oceans to lakes to ground and even in the sky as water vapour. We need water to carry out our daily activities. We cannot imagine our life without water. Rainwater is the main source of water on Earth.
Uses of water :
- Water is used for agriculture for the purpose of irrigation.
- Water is used for washing clothes, drinking, bathing, cleaning etc.
- It is also used in watering plants.
- Water is used in industries for power generation.
- One of the important uses of water is fire extinction.
Impurities in water:
Substances such as mud, sand, dirt and germs make water unfit for use. These are called impurities. Impurities are of two types:
- Soluble impurities : impurities that dissolve in water. E.g: Salt
- Insoluble impurities : impurities that do not dissolve in water. E.g: sand and mud
Solution
A solution is a mixture in which substances are completely dissolved. It can be defined as a special type of mixture where two or more substances are combined in such a way that they evenly spread and mix with each other.
Example: sugar mixed with water becomes a solution.
2 Substance to make Solution
The two substances that make a solution are:
- Solute
- Solvent
Solute:
- The substance which is soluble in other substances is called a solute.
- Solute is the component of the solution which is present in small amounts.
Solvent:
- a substance in which the solute is dissolved is called a solvent.
- Solvent is the component of solution which is present in large amounts.
Let us take an example to understand about solute and solvent:
When we mix salt and water it completely dissolves with each other and forms a solution. Here, salt is the solute and water is the solvent.
Removing Insoluble Impurities
Different methods are used to remove various soluble and insoluble impurities from water. Some substances like stones, mud,and sand do not dissolve in liquid(say water) and are called insoluble substances. We can separate the insoluble substances from water by sedimentation followed by decantation and filtration.
Sedimentation and decantation
when we mix mud in water, the color of the water gets changed. After some time we will see that the insoluble particles of mud settle down at the bottom. These are called sediments. This process of separating insoluble substances is called sedimentation.
Sedimentation steps
- Collect impure water containing mud in a beaker.
- Keep the water undisturbed for some time
- After some time, the mud settles down at the bottom of the beaker and is called sediment.
After the insoluble sediments have settled down, the clear water can be removed into another container. This process is called decantation.
Filtration: filtration is a process in which water containing insoluble substances are poured into a funnel having a cone of filter paper. Insoluble substances are caught in the filter paper and the clear water passes through it and gets collected in a vessel called a filtrate. This is a better process of separation than sedimentation and decantation.
Removing Soluble Impurities
It is more difficult to remove soluble impurities than insoluble ones. We can separate soluble substances from water by evaporation and distillation.
Boiling: in this process the soluble substances can be separated by heating the water(liquid). When all the water evaporates we will get the substance. Water is lost during the process of boiling.
Example: sugar and water solution is heated till the water evaporates leaving behind sugar particles.
Distillation: It is a method of purifying water generally performed in laboratories. The water collected after this process is called distilled water. It is free of any impurities. It is used in car batteries and medicines.
Drinking water
For a healthy living clean drinking water is to be consumed. Drinking water needs to be purified before consumption because impure water can lead to a variety of diseases.
Three methods are usually used for treatment of water:
- Screening : screening is a wastewater pre-treatment. In the process of screening the water is taken from lakes, rivers and passed through screens that remove large impurities like stones, leaves, plastics etc.
- Sedimentation: It is a process that removes solids that float and settle in the water. Water is collected in large tanks and left undisturbed for a few days. The heavier impurities settle down at the bottom. This waste is removed periodically. Chemicals like alum are added to purify water.
- Filtration: after the process of sedimentation, the water is filtered through sand beds to filter out small insoluble particles.
- Chlorination : Next, chlorine is added in small amounts to disinfect the water . The water is now safe for drinking.
-
Methods of purifying water at home – to make it fit for drinking
- Boil the water for about 15 mins to kill the germs and filter it.
- Add water purification or disinfecting tablets like chlorine dioxide in water to filter water.
- Use a water filter to store drinking water.
- Add potassium permanganate crystals in wells to clean water.
- Small amount of bleach can be used to clean water.

These types of websites are really helpful . I really love this, Good job 👍
Extremely good.
Thanks Jasneet