Table of Contents
Chapter 1- Matter In Our Surroundings – Class 9 – Textbook Question and answers
Which of the following are matter?
Given- Chair, air, love, smell, hate, almonds, thought, cold, lemon water, smell of perfume.
Answer-
Matter is anything that has mass and occupies space. It exists in different physical states, such as solid, liquid and gas.
From the given options, the following are considered as matter-
- Chair – A solid object that has mass and occupies space.
- Air – A mixture of gases that has mass and fills the space of its container.
- Almonds – These are solid and take up space, making them matter.
- Lemon water – A liquid that has a definite volume and takes the shape of its container.
The following are not matter because they do not have mass or occupy space-
- Love, hate, thought – These are emotions or feelings, which cannot be physically measured.
- Smell, smell of perfume, cold – These are effects or sensations rather than substances with mass.
Thus, only chair, air, almonds and lemon water are matter.
Also Check – Chapter 1 -MATTER IN OUR SURROUNDINGS -Class 9 – Simplified notes
Why does the smell of hot sizzling food reach you several metres away, but to get the smell from cold food you have to go close?
Answer-
The smell of food reaches us due to the process of diffusion. Diffusion is the movement of particles from a region of higher concentration to a region of lower concentration without any external force.
- When food is hot and sizzling, the kinetic energy of its aroma particles increases due to heat. These high-energy particles move faster, mix with air and spread over a larger area, allowing the smell to be detected from a distance.
- In contrast, when food is cold, the aroma particles have lower kinetic energy and do not move as quickly. As a result, the smell remains concentrated near the food and one has to go closer to detect it.
This observation proves that particles of matter are continuously moving and their movement increases with temperature.
A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Answer-
This observation demonstrates the property that particles of matter have space between them and are loosely held in liquids compared to solids.
- Water is a liquid and its particles have a weaker force of attraction than solids. This allows them to move freely and adjust their positions.
- Because of this, when a diver jumps into the pool, the water particles move aside, allowing the diver to pass through.
- Liquids also have fluidity, meaning they can flow and change shape, unlike solids, which retain a fixed shape.
Thus, the ability of a diver to cut through water proves that there is space between particles of matter and liquids have a lower force of attraction compared to solids.
What are the characteristics of the particles of matter?
Answer-
Particles of matter exhibit the following characteristics-
- Particles of matter have space between them
- Different substances have different amounts of space between their particles.
- Example- When sugar or salt is dissolved in water, it disappears because its particles fill the spaces between water molecules.
- Particles of matter are continuously moving
- All particles of matter are in constant motion and have kinetic energy.
- Example- The smell of perfume spreads across a room without stirring because its particles diffuse in the air due to their motion.
- Particles of matter attract each other
- The force of attraction between particles holds them together.
- Example- Solids have strong forces of attraction between particles, which is why an iron rod is hard and difficult to break, while liquids and gases have weaker attractions.
These characteristics explain the different states of matter and their properties, such as diffusion, compressibility and fluidity.
Arrange the following in order of increasing density – air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Answer-
Density is defined as the mass per unit volume of a substance. It is calculated using the formula-
Density = Mass / Volume
The order of increasing density for the given substances is-
Air < Exhaust from chimneys < Cotton < Water < Honey < Chalk < Iron
- Air has the least density because gas particles are widely spaced.
- Exhaust from chimneys consists of gases and small solid particles, making it slightly denser than air.
- Cotton is a solid but has a lot of air trapped inside, making it less dense than water.
- Water has a fixed density of 1 g/cm³ and is denser than cotton.
- Honey is more viscous and contains sugar molecules, making it denser than water.
- Chalk is a solid with tightly packed particles, so it has more density than honey.
- Iron has the highest density among the given substances, as metals have tightly packed particles with a high mass.
Thus, the correct order from lowest to highest density is-
Air → Exhaust from chimneys → Cotton → Water → Honey → Chalk → Iron.
Also Check – Rapid Revision –Chapter 1- Matter In Our Surroundings – Class 9 Science
(a) Tabulate the differences in the characteristics of states of matter.
| Property | Solid | Liquid | Gas |
| Shape | Fixed shape | No fixed shape; takes the shape of the container | No fixed shape; fills the entire container |
| Volume | Fixed volume | Fixed volume | No fixed volume; expands to fill the container |
| Compressibility | Negligible (almost incompressible) | Slightly compressible | Highly compressible |
| Intermolecular Spaces | Very small (particles are tightly packed) | More than solids but less than gases | Very large (particles are far apart) |
| Intermolecular Forces | Strongest | Weaker than solids but stronger than gases | Weakest |
| Fluidity | Cannot flow | Can flow | Can flow |
| Density | Highest | Lesser than solids | Least |
(b) Comment upon the following-
- Rigidity- The ability of a substance to retain its shape and not deform under external force. Solids are rigid due to strong intermolecular forces, while liquids and gases are not.
- Compressibility- The ability to decrease volume under pressure. Gases are highly compressible, while solids and liquids have very low compressibility.
- Fluidity- The ability of a substance to flow. Liquids and gases are fluids, while solids are rigid and cannot flow.
- Filling a Gas Container- Gases do not have a fixed volume and expand to occupy the entire container.
- Shape- Solids have a definite shape, whereas liquids take the shape of their container and gases completely fill the container.
- Kinetic Energy- The energy of particles due to their motion. It is least in solids, higher in liquids and highest in gases.
- Density- The mass per unit volume of a substance. Solids generally have the highest density, followed by liquids, while gases have the lowest.
Give Reasons-
(a) A gas fills completely the vessel in which it is kept.
Gases do not have a fixed shape or volume. The particles of a gas move randomly in all directions at high speeds due to weak intermolecular forces. This random motion allows gas particles to spread out and completely occupy any container.
(b) A gas exerts pressure on the walls of the container.
Gas particles are in constant motion and collide with each other and with the walls of the container. These collisions exert force on the walls, creating pressure. The more the particles move, the higher the pressure exerted.
(c) A wooden table should be called a solid.
A wooden table has a definite shape and volume. Its particles are tightly packed, making it rigid. It cannot flow like liquids or gases, which are characteristics of solids.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Air is a gas and its particles are widely spaced with weak intermolecular forces, allowing easy movement. In contrast, wood is a solid with tightly packed particles and strong intermolecular forces, making it difficult to break or pass through without applying significant force.
Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Ice is a solid form of water but floats on liquid water due to its lower density. This is because, when water freezes, its molecules arrange in a rigid hexagonal structure with more empty space, making ice less dense than liquid water. Since less dense substances float on denser substances, ice floats on water.
Also Check – 82 Reasoning-Based Questions & Answers-Matter In Our Surroundings -Class 9 Science
Convert the following temperature to the Celsius scale-
To convert Kelvin (K) to Celsius (°C), use the formula-
°C = K – 273
(a) 300 K
300 – 273 = 27°C
(b) 573 K
573 – 273 = 300°C
What is the physical state of water at-
(a) 250°C – Water exists as steam (gas) at this temperature, since it is above the boiling point (100°C).
(b) 100°C – Water exists as both liquid and gas. At 100°C, water starts to boil, meaning it exists in equilibrium between liquid and gaseous states.
For any substance, why does the temperature remain constant during the change of state?
When a substance undergoes a change of state (solid to liquid or liquid to gas), the temperature remains constant even though heat is continuously supplied. This happens because the heat energy supplied is used to overcome the forces of attraction between the particles rather than increasing their kinetic energy.
- During melting, the heat energy is used to break the strong intermolecular bonds in the solid, allowing it to turn into a liquid. This absorbed heat is called the latent heat of fusion.
- During boiling, the heat energy is used to convert the liquid into gas by overcoming the forces of attraction between the particles. This is known as the latent heat of vaporization.
Since this energy is hidden and does not raise the temperature, it is called latent heat. Thus, the temperature remains constant until the entire substance has changed its state.
Suggest a method to liquefy atmospheric gases.
To liquefy atmospheric gases such as oxygen, nitrogen and carbon dioxide, we need to lower the temperature and increase the pressure.
The steps to liquefy gases are-
- Cooling- The gas is first cooled to a very low temperature using refrigeration or expansion techniques. When the temperature is lowered, the kinetic energy of the gas particles decreases, making them move slower.
- Compression- The gas is then subjected to high pressure. This forces the particles to come closer, increasing their intermolecular forces and leading to condensation into a liquid state.
For example, liquid oxygen (LOX) and liquid nitrogen (LIN) are produced using this method and stored in insulated containers.
Also Check – Chapter 1- Matter In Our Surroundings – Class 9 Science – Long Question and Answers
Why does a desert cooler cool better on a hot dry day?
A desert cooler works on the principle of evaporation. When water in the cooler evaporates, it absorbs heat from the surroundings, leading to a cooling effect.
- On a hot dry day, the air has low humidity, which means it can absorb more water vapor.
- Since the rate of evaporation increases when humidity is low, more heat is absorbed from the air, making the cooling effect stronger.
- If the air is already humid (as in rainy weather), the evaporation rate decreases and the cooling effect is reduced.
Thus, a desert cooler is more effective in hot and dry weather because faster evaporation leads to better cooling.
How does the water kept in an earthen pot (matka) become cool during summer?
An earthen pot cools water due to evaporative cooling.
- The walls of an earthen pot are porous, allowing some water to seep through to the outer surface.
- This water evaporates into the air, taking away heat energy from the remaining water inside the pot.
- As a result, the temperature of the water inside the pot decreases, making it cooler than the surrounding air.
This natural cooling process is similar to how sweating helps in cooling the human body.
Also Check – The Science of Evaporation – Process, Factors, and Applications
Why does our palm feel cold when we put some acetone, petrol, or perfume on it?
When acetone, petrol, or perfume is applied to the skin, it evaporates quickly.
- These liquids have low boiling points and evaporate rapidly at room temperature.
- During evaporation, they absorb heat energy from our palm to change into vapor.
- This heat loss from our skin makes us feel cold.
This effect is similar to how sweat evaporates from our body, cooling it down.
Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
We can sip hot tea or milk faster from a saucer because of the increase in surface area.
- A saucer has a larger surface area compared to a cup.
- When a liquid spreads over a larger area, more particles are exposed to air, which increases the rate of evaporation.
- Faster evaporation means the liquid cools down more quickly, making it easier to sip.
In contrast, a cup has a smaller surface area, so the cooling process is slower.
What type of clothes should we wear in summer?
In summer, we should wear light-colored, loose and cotton clothes because-
- Cotton absorbs sweat-
- Cotton fabric absorbs sweat from the body and allows it to evaporate.
- This evaporation absorbs heat from the skin, helping in cooling the body.
- Light colors reflect heat-
- Dark-colored clothes absorb more heat, making us feel hotter.
- Light-colored clothes reflect sunlight, keeping us cooler.
- Loose-fitting clothes allow air circulation-
- Loose clothes allow air to pass through, helping in the evaporation of sweat, which keeps the body cool.
Thus, cotton, light-colored and loose clothes are best suited for summer.
Chapter 1- Matter In Our Surroundings – Exercises – Question and Answers
1. Convert the following temperatures to the Celsius scale.
To convert temperature from Kelvin (K) to Celsius (°C), use the formula-
°C = K – 273
(a) 293 K
293 – 273 = 20°C
(b) 470 K
470 – 273 = 197°C
2. Convert the following temperatures to the Kelvin scale.
To convert temperature from Celsius (°C) to Kelvin (K), use the formula-
K = °C + 273
(a) 25°C
25 + 273 = 298 K
(b) 373°C
373 + 273 = 646 K
Also Check – Chapter 1- Matter In Our Surroundings – Class 9 Science – Solved MCQs
3. Give reasons for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
Naphthalene undergoes sublimation, which means it directly changes from a solid to a gas without turning into a liquid. The molecules of naphthalene gain energy from their surroundings and get converted into gas, mixing with air. This is why the naphthalene balls shrink and eventually disappear over time without leaving any solid residue.
(b) We can get the smell of perfume sitting several meters away.
Perfume contains volatile substances that evaporate easily. When sprayed, the perfume particles diffuse rapidly in air due to their high kinetic energy. The movement of air helps in spreading these particles over a large distance, allowing us to smell the perfume even from several meters away.
Also Check – Sublimation- How Solids Turn Into Gas Without Melting
4. Arrange the following substances in increasing order of forces of attraction between the particles- water, sugar, oxygen.
The intermolecular forces of attraction are strongest in solids, weaker in liquids and weakest in gases.
Increasing order of forces of attraction-
Oxygen (gas) < Water (liquid) < Sugar (solid)
- Oxygen has the weakest intermolecular forces as its particles are widely spread.
- Water has moderate intermolecular forces, allowing its particles to flow but still stay close.
- Sugar has the strongest intermolecular forces, keeping its particles tightly bound in a fixed structure.
5. What is the physical state of water at-
(a) 25°C → Liquid
At 25°C, water is in its normal liquid state as it is above freezing point and below boiling point.
(b) 0°C → Both solid and liquid
At 0°C, water is at its melting/freezing point, meaning it can exist as both ice and liquid water in equilibrium.
(c) 100°C → Both liquid and gas
At 100°C, water is at its boiling point, meaning it can exist as both liquid and steam in equilibrium.
Also Check – Latent Heat of Fusion Formula- Easy Explanation with Formula & Examples
Also Check – What is Latent Heat? Fusion & Vaporization Explained with Real-Life Applications
6. Give two reasons to justify the following statements.
(a) Water at room temperature is a liquid.
- Water has a definite volume but no fixed shape. It takes the shape of the container, which is a property of liquids.
- The intermolecular forces in water are strong enough to keep it in a liquid state but not strong enough to keep it in a fixed shape like a solid.
(b) An iron almirah is a solid at room temperature.
- An iron almirah has a fixed shape and volume, which are characteristics of solids.
- The particles in iron are tightly packed with strong intermolecular forces, making it rigid and incompressible.
Also Check – Matter In Our Surroundings – Worksheet with Answer Key
7. Why is ice at 273 K more effective in cooling than water at the same temperature?
Ice at 273 K (0°C) is more effective in cooling than water at the same temperature because-
- Latent heat of fusion- When ice melts, it absorbs additional heat energy (latent heat of fusion) from its surroundings without raising its temperature. This extra absorption of heat cools the surrounding area more effectively than water.
- Water at 273 K does not absorb extra heat- Since water at 273 K is already in liquid form, it does not need to absorb latent heat, making it less effective in cooling.
8. What produces more severe burns, boiling water or steam?
Steam produces more severe burns than boiling water because-
- Steam contains additional heat energy (latent heat of vaporization)- Steam at 100°C carries extra heat energy, which is released when it condenses back to liquid water on contact with the skin. This heat release increases the severity of burns.
- Boiling water does not have latent heat of vaporization- Boiling water at 100°C only transfers heat due to temperature difference, whereas steam transfers additional latent heat, making it more dangerous.
9. Name A, B, C, D, E and F in the following diagram showing change in its state.
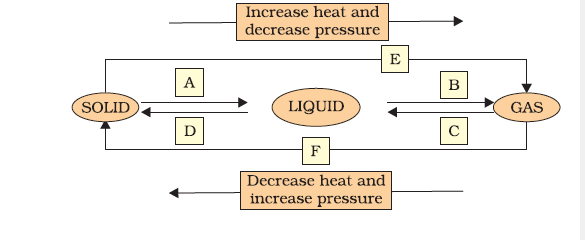
The diagram represents the interconversion of states of matter–
- A → Melting (Solid to Liquid)
- B → Evaporation (Liquid to Gas)
- C → Condensation (Gas to Liquid)
- D → Freezing (Liquid to Solid)
- E → Sublimation (Solid to Gas)
- F → Deposition (Gas to Solid)
Also Chekc – NCERT Exemplar Solutions- Class 9 Science Chapter – 1 – Matter in Our Surroundings

