Table of Contents
What is Evaporation?
Evaporation is the process by which a liquid turns into a gas at temperatures lower than its boiling point. It happens at the surface of a liquid, where molecules with enough energy escape into the air as vapor.
Everyday Examples of Evaporation
- A wet towel drying in the sun.
- Water in an open glass disappearing over time.
- Sweat evaporating from the skin, keeping the body cool.
- Puddles drying up after it rains.
How Does Evaporation Work?
Evaporation is a process at the molecular level, where liquid molecules gain energy and escape into the air as vapor. This occurs without the need for boiling and happens only at the surface of the liquid. To fully understand evaporation, we need to examine how molecular motion, energy transfer and intermolecular forces contribute to this phenomenon.
1. Molecular Motion- The Foundation of Evaporation
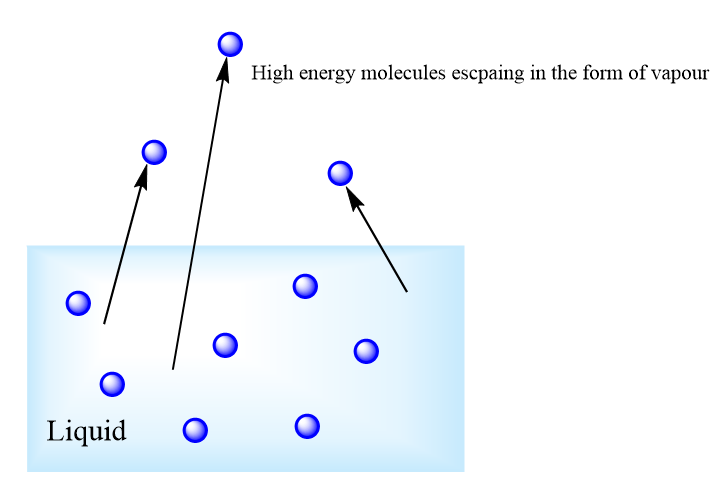
All matter, including liquids, is made up of tiny molecules that are constantly moving due to thermal energy. However, not all molecules move at the same speed.
- In a liquid, molecules collide with each other, transferring energy in the process.
- Some molecules move fast, while others move slowly.
- These differences in movement are due to kinetic energy, which is the energy of motion.
At any given moment-
- Some molecules have high energy and move rapidly.
- Others have low energy and move more slowly.
This variation in energy levels plays a crucial role in evaporation because only high-energy molecules can escape the liquid phase.
2. Energy Gain- How Molecules Absorb Heat
For a liquid molecule to transition into the gaseous phase, it must gain enough energy to break free from the attraction of surrounding molecules.
Where Does the Energy Come From?
- Heat from the Sun or Surroundings– Sunlight or warm air transfers energy to the liquid, increasing the kinetic energy of its molecules.
- Collisions Between Molecules– Faster-moving molecules transfer energy to slower ones when they collide. This can give some molecules the extra boost they need to evaporate.
- External Heat Sources– If placed near a heat source like a warm surface, a flame, or even body heat, the liquid absorbs energy more quickly, accelerating evaporation.
Since molecules do not all gain energy equally, only the ones with the highest kinetic energy can escape. This is why evaporation is a gradual process.
Also Check – 15 Surprising and Real Facts About Evaporation
3. Escape- Overcoming Intermolecular Forces
Even if a molecule has high energy, it must overcome the forces of attraction holding it within the liquid. These forces are called intermolecular forces.
- Liquids have cohesive forces, meaning molecules are attracted to each other.
- For a molecule to leave the liquid and become vapor, it needs to break free from these forces.
- This requires a molecule to have sufficient kinetic energy to overcome this attraction.
Molecules at the surface of the liquid are more likely to escape because-
- They are exposed to air, reducing the number of molecules pulling them back into the liquid.
- They only need to break free from forces below them, unlike molecules inside the liquid, which are surrounded on all sides.
Once a high-energy molecule successfully escapes, it enters the air as water vapor.
4. Continuous Evaporation- Why Liquids Keep Evaporating
Evaporation does not stop after the first molecules escape. Instead, it continues in a self-sustaining process–
- High-energy molecules leave the liquid, reducing the average energy of the remaining liquid.
- More molecules gain energy through heat absorption and molecular collisions.
- Another batch of high-energy molecules escapes and the cycle repeats.
This explains why-
- A cup of water left out gradually disappears over time.
- Sweat continues to evaporate from the skin as long as heat is available.
- Puddles dry up after rain, even on days without direct sunlight.
5. The Role of Partial Pressure and Saturation
Once molecules escape into the air as vapor, they do not disappear. Instead, they mix with the surrounding air, creating water vapor pressure.
- If the air has little moisture, it can hold more evaporated molecules, allowing evaporation to continue.
- If the air is already full of moisture (high humidity), fewer molecules can escape and evaporation slows down.
This is why evaporation happens faster in dry conditions and slower in humid conditions
6. Why Evaporation Happens Without Boiling
Both evaporation and boiling involve liquid turning into gas, but they are fundamentally different processes–
| Evaporation | Boiling |
| Happens at all temperatures | Happens at boiling point (e.g., 100°C for water) |
| Occurs only at the surface of the liquid | Occurs throughout the entire liquid |
| Slow process | Fast process |
| No visible bubbles | Bubbles form as vapor escapes rapidly |
The key reason evaporation happens without reaching the boiling point is that only some molecules gain enough energy to escape, rather than the entire liquid turning into gas at once.
Factors That Affect Evaporation
Evaporation is influenced by multiple factors, each playing a role in how quickly a liquid turns into vapor. These factors impact the energy levels of molecules, the number of molecules that can escape from the liquid’s surface and the surrounding environmental conditions.
1. Temperature- Energy for Evaporation
Concept- More Heat = More Energy
- Higher temperatures increase the kinetic energy of the liquid’s molecules.
- Molecules move at different speeds and some move faster than others.
- When a molecule at the surface gains enough energy, it can break free from the liquid and turn into vapor.
- More heat means more molecules have the necessary energy to escape, increasing the rate of evaporation.
Example- Drying Clothes on a Hot Day
- On a hot day, the sun heats the water molecules in wet clothes.
- This added energy allows more water molecules to escape into the air.
- As a result, the clothes dry faster than on a cold day.
Scientific Connection- Latent Heat of Vaporization
- Every liquid needs a certain amount of latent heat to transition from a liquid to a gas.
- Water has a high latent heat of vaporization, meaning it requires more energy to evaporate compared to substances like alcohol or acetone.
2. Surface Area- More Exposure, Faster Evaporation
Concept- A Wider Area Allows More Molecules to Escape
- Evaporation happens at the surface of a liquid.
- If the surface area is larger, more molecules have access to air, leading to faster evaporation.
- A smaller surface area means fewer molecules are exposed at any given time, slowing the process.
Example- A Spilled Drink vs. Water in a Cup
- A spilled drink on a table dries faster than the same amount of liquid left in a glass.
- The thin layer of liquid in the spill has more surface area exposed to air, allowing more molecules to evaporate at once.
- In contrast, the liquid in a cup has a smaller exposed surface, limiting evaporation.
Scientific Connection- Evaporation Rate and Surface Exposure
- Increasing surface area is commonly used in cooling devices like earthen pots and industrial evaporators.
- Wet clothes spread out dry faster than those folded because of increased surface exposure.
3. Humidity- More Water in the Air Slows Evaporation
Concept- Air Can Only Hold So Much Water
- The air around us contains water vapor.
- The higher the humidity, the more saturated the air is with water molecules.
- If the air is already full of moisture, it reduces the space for new vapor molecules from the liquid.
- This slows down evaporation because fewer molecules can leave the liquid.
Example- Clothes Dry Slower on a Humid Day
- On a humid day, the air is already filled with water vapor.
- The moisture in the clothes struggles to evaporate because there is no room for more water in the air.
- In contrast, on a dry, sunny day, the air can absorb more moisture, making evaporation faster.
Scientific Connection- Relative Humidity and Saturation
- Relative humidity is the amount of moisture in the air compared to the maximum amount it can hold.
- At 100% humidity, evaporation stops completely because the air is fully saturated.
4. Air Movement (Wind)- Blowing Away Water Vapor
Concept- Moving Air Removes Evaporated Molecules
- When a molecule evaporates, it stays near the surface for a short time before mixing into the air.
- If no wind is present, the air near the surface becomes saturated with vapor, reducing the evaporation rate.
- Wind or air movement removes these vapor molecules, making space for new ones to evaporate.
- This keeps evaporation going at a faster rate.
Example- Fans Help Dry Sweat Faster
- When we sweat, the moisture on our skin evaporates to cool us down.
- On a windy day or when using a fan, sweat evaporates faster because the moving air carries away the evaporated moisture.
- Without air movement, the humidity near the skin increases, slowing evaporation.
Scientific Connection- Wind and Evaporative Cooling
- In hot, dry regions, desert coolers work by forcing air over water-soaked pads, speeding up evaporation and cooling the air.
- Farmers sometimes spray water on crops and wind helps dry excess moisture to prevent mold and rot.
5. Nature of the Liquid- Molecular Attraction Affects Evaporation
Concept- Some Liquids Evaporate Faster Than Others
- Not all liquids evaporate at the same speed because different liquids have different molecular forces.
- Liquids with weak molecular forces evaporate faster.
- Liquids with strong molecular attraction (like water) take longer to evaporate because more energy is needed to break these bonds.
Example- Alcohol Evaporates Faster Than Water
- Alcohol evaporates very quickly compared to water.
- This is because alcohol molecules have weaker intermolecular forces, requiring less energy to escape into the air.
- Water has strong hydrogen bonds, making it slower to evaporate.
Scientific Connection- Volatility and Intermolecular Forces
- Volatile liquids like acetone and alcohol have low boiling points and weak intermolecular forces, causing them to evaporate quickly.
- Non-volatile liquids like oil take much longer to evaporate because their molecules strongly attract each other.
Evaporation Causes Cooling
One of the most important effects of evaporation is that it causes cooling. This happens because when a liquid evaporates, it absorbs heat energy from its surroundings, lowering the temperature.
How Does Evaporation Cause Cooling?
- Heat Absorption–
- When a liquid evaporates, its molecules need energy to escape into the air as vapor.
- This energy comes from the surrounding environment, including the surface of the liquid and nearby objects.
- Loss of High-Energy Molecules–
- The fast-moving molecules with the most energy evaporate first.
- The remaining molecules have lower energy, reducing the temperature of the liquid and its surroundings.
- Cooling Effect–
- Since energy (heat) is taken away during evaporation, the temperature drops, creating a cooling effect.
Examples of Evaporation Causing Cooling
- Sweating and Body Cooling–
- When we sweat, the moisture on our skin absorbs body heat and evaporates.
- This cools the skin and helps regulate body temperature.
- On humid days, sweat evaporates slower, making it harder to cool down.
- Cooling After a Bath–
- When stepping out of a shower or swimming pool, water on the skin evaporates.
- This process absorbs heat from the body, causing a slight chill.
- Hand Sanitizer and Alcohol Cooling–
- When applying hand sanitizer, it feels cool because alcohol evaporates quickly, taking heat from the skin.
- Cooling Effect of Wet Clothes–
- Clothes dry when water evaporates, but during the process, they feel cooler due to heat absorption.
- Earthen Pots (Matkas) for Water Cooling–
- Traditional clay pots have tiny pores that allow water to seep out and evaporate.
- This evaporation absorbs heat, cooling the water inside.
- Desert Coolers–
- Air coolers use evaporation to cool down rooms.
- Water in the cooler evaporates, reducing the surrounding temperature.
Scientific Explanation (Latent Heat of Vaporization)
Evaporation is linked to a concept called latent heat of vaporization, which is the energy required to turn a liquid into a gas without increasing its temperature.
- Water has a high latent heat of vaporization.
- This means it absorbs a large amount of heat when evaporating, making it effective for cooling.
For example-
- When sweat evaporates, it absorbs body heat but remains at the same temperature.
- This helps the body lose excess heat without directly raising air temperature.
Evaporation vs. Boiling
| Evaporation | Boiling |
| Happens at any temperature | Happens at a fixed boiling point (100°C for water) |
| Only at the surface | Throughout the liquid |
| Slow process | Fast process |
| No bubbles | Bubbles form |
Example- A puddle dries up over time (evaporation), but water in a pot boils quickly on a stove.


