What are the Characteristics of the Particles of Matter ?
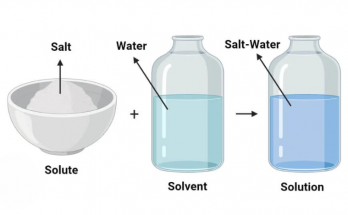
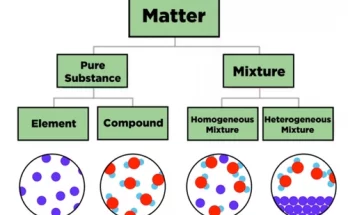
Particles of matter are extremely small, constantly in motion and have spaces between them. They possess mass, attract each other and can diffuse naturally. These fundamental characteristics explain how solids, liquids and gases behave differently. Understanding these properties is essential in physics and chemistry for grasping the nature and structure of all matter.
What are the Characteristics of the Particles of Matter ? Read More