Sublimation- How Solids Turn Into Gas Without Melting
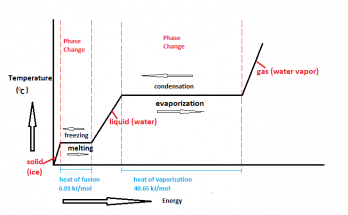
Sublimation is when a solid turns straight into gas without melting into liquid. This happens with things like dry ice, mothballs, and even snow in cold places. It’s used in printing, food drying, and space science. Learn how it works and where you see it in daily life!
Sublimation- How Solids Turn Into Gas Without Melting Read More