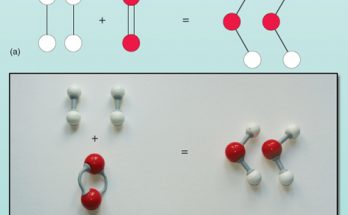
Why are decomposition reactions called the opposite of combination reactions? Write an equation for this reaction.
Decomposition reactions are called opposite of combination reactions due to their reverse processes. In a decomposition reaction, compounds break down into simpler substances, contrasting with combination reactions where substances merge to form compounds. For example, heating calcium carbonate (CaCO₃) decomposes it into calcium oxide (CaO) and carbon dioxide (CO₂). This equation demonstrates the reversal of processes in decomposition reactions compared to combination reactions.
Why are decomposition reactions called the opposite of combination reactions? Write an equation for this reaction. Read More