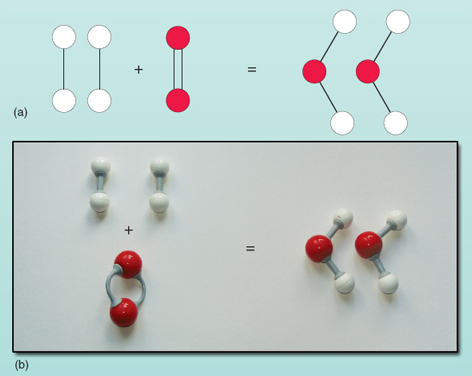
Question: What is a balanced chemical equation? Why should chemical equations be balanced?
Answer:
A balanced chemical equation is a representation of a chemical reaction where the number of each type of atom is equal on both sides of the equation, ensuring the mass is conserved according to the law of conservation of mass. The process of adjusting the coefficients of the reactants and products to achieve this balance is known as the balancing of equations.
Balancing Chemical Equations: An Example
Consider the reaction between hydrogen (H2) and oxygen (O2) to form water (H2O). Initially, the equation might look unbalanced as:
H2+O2→H2O
- In this reaction H2 and O2 are reactants where H20 is the product.
- Count the number of hydrogen atoms and oxygen atom in reactant and product
- Number of hydrogen atoms in reactants is 2 and in product is 2
- Number of oxygen atoms in reactants is 2 and in product is 1
- The number of hydrogen atoms is equal on both sides, but the number of oxygen atoms are unequal. To have two oxygen atom on right side we multiply H2O and write 2 H2O
2H2+O2→2H2O
Now, the equation is balanced with equal numbers of hydrogen and oxygen atoms on both sides.
Importance of Balancing Chemical Equations:
- Conservation of Mass: Balancing ensures compliance with the law of conservation of mass, which states that mass cannot be created or destroyed in a chemical reaction. The total mass of reactants must equal the total mass of products.
- Stoichiometry: A balanced equation accurately represents the stoichiometry of the reaction, providing the exact proportions of reactants and products involved. This is crucial for calculating the amounts of substances required or produced in a reaction.
- Physical States and Heat Changes: A balanced chemical equation also conveys information about the physical states of reactants and products (solid, liquid, gas, or aqueous) and about heat changes during the reaction, indicating whether the reaction is endothermic or exothermic.